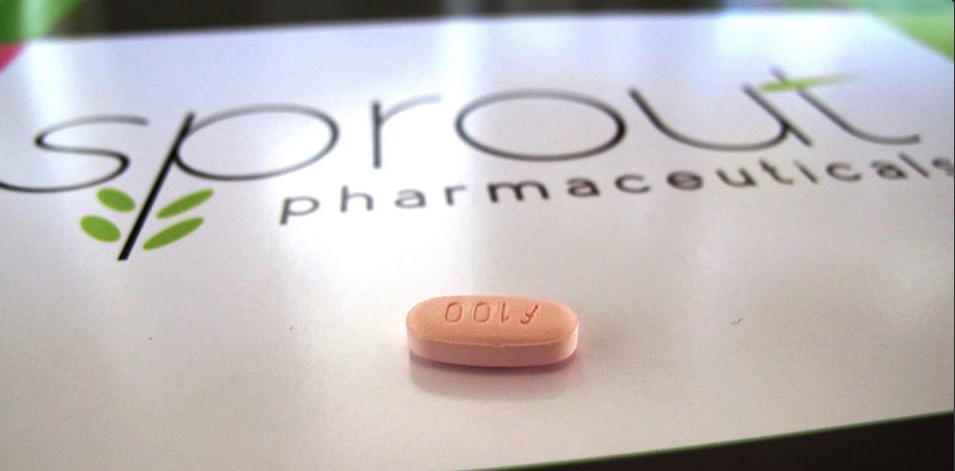
Gentleman, Ladies “Viagra” Has Been Approved by FDA
18 Aug, 2015
The Food and Drug Administration on Tuesday approved the first prescription drug designed to boost sexual desire in women, a milestone long sought by a pharmaceutical industry eager to replicate the blockbuster success of impotence drugs for men.
But stringent safety measures on the daily pill called Addyi mean it will probably never achieve the sales of Viagra, which has generated billions of dollars since the late 1990s.
The drug’s label will bear a boxed warning – the most serious type – alerting doctors and patients to the risks of dangerously low blood pressure and fainting, especially when the pill is combined with alcohol. The same problems can occur when taking the drug with other commonly prescribed medications, including antifungals used to treat yeast infections.
“Patients and prescribers should fully understand the risks associated with the use of Addyi before considering treatment,” said Dr. Janet Woodcock, director of the FDA’s drug center, in a statement.
Under an FDA-imposed safety plan, doctors will only be able to prescribe Addyi after completing an online certification process that requires counseling patients about Addyi’s risks. Pharmacists will also need certification and will be required to remind patients not to drink alcohol while taking the drug.
Opponents of the drug say it’s not worth the side effects, which also include nausea, drowsiness and dizziness. They point out that the FDA rejected the drug twice, in 2010 and 2013, due to these risks.
Sprout Pharmaceutical’s drug is intended to treat women who report emotional stress due to a lack of libido. Its approval marks a turnaround for the FDA, which previously rejected the drug twice due to lackluster effectiveness and side effects. The decision represents a compromise of sorts between two camps that have publicly feuded over the drug for years.
On one side, Sprout and its supporters have argued that women desperately need FDA-approved medicines to treat sexual problems. On the other side, safety advocates and pharmaceutical critics warn that Addyi is a problem-prone drug for a questionable medical condition.
The search for a pill to treat women’s sexual difficulties has been something of a holy grail for the pharmaceutical industry. It was pursued and later abandoned by Pfizer, Bayer and Procter & Gamble, among others. But drugs that act on blood flow, hormones and other biological functions all proved ineffective.
Addyi, known generically as flibanserin, is the first drug that acts on brain chemicals that affect mood and appetite.
Women and their doctors will have to decide whether the drug’s modest benefits warrant taking a psychiatric pill on a daily basis.
Company trials showed women taking the drug generally reported one extra “sexually satisfying event” per month, and scored higher on questionnaires measuring desire.
AP
Image Poltico twitter
Mentioned In This Post:
About the author
Related Posts
-

They're Back with More Listings and More Drama
-

If You're Gonna Steal, Keep it Off The Gram
-
The New NFL Tiger King
-

Diddy's Making the Band is Back
-

Romeo Miller and the Ex's on the Beach
-

Mac Millers Cause Of Death Revealed
-

It's Over For College Basketball
-

Now Eight Kids Are Safe
-

No Charges in The Murder of Alton Sterling
-

The Obama's Are Artwork